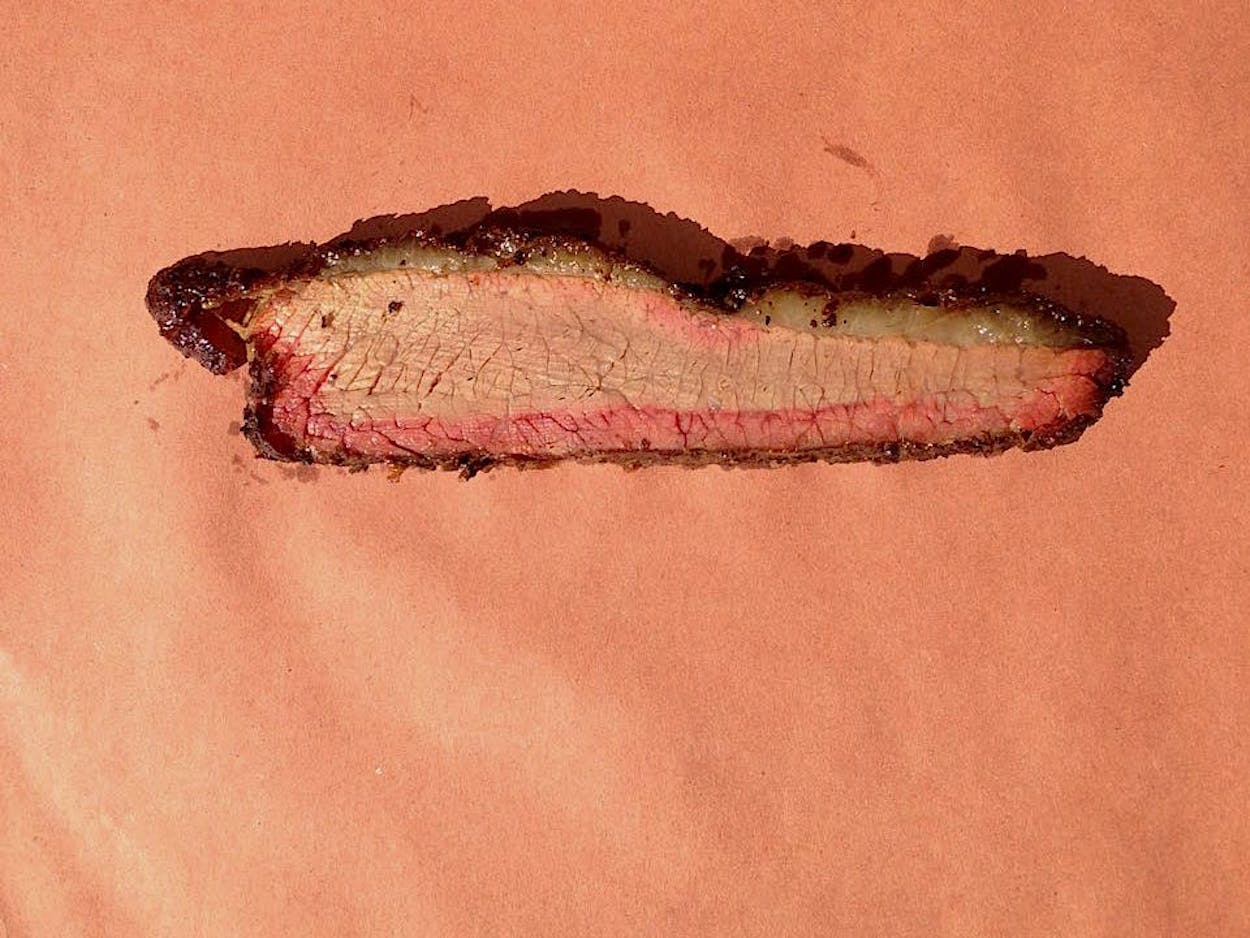
Let me drop a hard truth-bomb on you: as an indicator of well-cooked barbecue, the smoke ring is useless. Narcissus was less attached to his reflection than some barbecue cooks and critics are to that thin red line along the perimeter of smoked meats, but as with that Greek myth, worship of the smoke ring is mere vanity.
While I agree that a rosy halo gives a slice of brisket a more desirable appearance, I’ve eaten enough barbecue to not let it fool me into a pre-bite opinion. I’ve had dry, awful brisket with a well-endowed smoke ring, and one of the best, and smokiest briskets around is at The Slow Bone in Dallas that doesn’t have much of one. With his brisket on display on the cutting board, owner Jack Perkins gets plenty of questions about it from new customers. “Every now and then we’ll have someone who watches a lot of television who asks ‘Where is the smoke ring?'” If they’re asking after the meal, “The first thing we ask is ‘Was it delicious?'”
The smoke ring isn’t something Perkins chases in his restaurant’s cooking method. “We’re looking for tenderness and juiciness,” he told me. As part of achieving this, they let their briskets warm up a bit (but not above health department mandated temperatures) outside the cooler before they go on the smoker. This is a common tip for home barbecue cooks to help accelerate the cooking process (colder briskets take longer in the smoker). As it happens, it’s also the best way to kill the smoke ring before it has a chance to form.

To understand the value of the smoke ring—or lack there of—let’s examine the process by which it is created. Well, maybe “created” isn’t the right term. The smoke ring is already within the meat in the form of myoglobin. It’s the protein that makes raw meat red or pink. As the meat cooks, myoglobin turns brown, but if enough nitric oxide (NO) from the wood smoke condenses on your meat, it will bind with the still-red myoglobin and allow it to hold onto its color. So the smoke ring is really a remnant. It’s a biological marker of how quickly the meat cooked, and how much NO was able to stick to the meat before it turned brown throughout. (That’s the short version. See the footnote below for a more detailed explanation.)

Maybe you’re not interested in science, and you just want a big smoke ring. The meat you’ve chosen to cook is the first indicator of how much of that red color you can hang onto. The red is all from myoglobin (the hemoglobin in the blood left the meat long before in the slaughterhouse), and different species have different concentrations of myoglobin in their muscles. Animals like whales and seals that need to hold their breath for long periods have the most. Their meat is dark purple. Wild game is a bit lighter, but still a deep red. Then there’s the rosier red color of beef and even some heritage hog breeds. Commodity hogs are a much lighter pink, but not as light as white meat turkey or chicken. That boneless, skinless chicken breast isn’t getting a deep red smoke ring.

The fuel you choose to cook with determines the amount of NO that can be produced. Dr. Greg Blonder, a professor of design and product engineering at Boston University and frequent contributor to Amazingribs.com, has done considerable research in this field, and he has shared his findings on his website. According to Blonder, wood bark contains more nitrogen (therefore, more potential NO) than either sapwood (the newer, outer part of a stem or branch) or the heartwood (the inner portion), and charcoal briquettes contain even more. As cut wood dries, or seasons, it loses nitrogen as well, so green wood is better than seasoned wood if a smoke ring is the goal.

Sawdust doesn’t work so well at creating NO. It’s often added to a charcoal fire for smoke flavor, but doesn’t have much of a chance to combust before turning to smoke. Dr. Jeff Savell at Texas A&M said this was a problem with their industrial-sized smoker that uses sawdust for the smoke flavor. “In our smokehouse that produces smoke, we don’t get a smoke ring,” he told me. He added that some customers questioned him about the smoke ring, so they experimented with manufacturing one by using a rub additive (more on that later). According to Savell, it “looks fake. You cut it open and it looks like someone painted it on.”
You then have to consider the temperature of the meat being cooked, and of the smoker. Myoglobin begins to turn brown at around 140 degrees, and will be brown throughout around 160 degrees internal temperature. You want those NO particles getting into the meat before it gets to that temperature. Cold meat into a low-temperature smoker is the best method. Dr. Blonder even went so far as to put a frozen tenderloin onto his smoker, which resulted in an impressive smoke ring. Keeping the smoking temperature low also allows the meat to remain below 140 for a longer period. That’s where a thermometer controlled gas-fired rotisserie can help.
How you treat the surface of the meat also makes a difference. NO more readily adheres to a tacky surface, so the meat should be moist. Dry meat won’t hold onto the NO. Spraying or mopping the meat during cooking can help with this. But the ring can be compromised if you use an acidic substances, like vinegar or lemon juice, to add moisture. Dr. Blonder recognized this and added some base ingredients to his rub like baking soda (not recommended) to get a deeper ring. Chasing the smoke ring can become a crazy game.

To review, you can enhance the smoke ring in a number of ways. Start with frozen meat, on a thermometer-controlled rotisserie fueled by charcoal briquettes and green wood. Spray with water to keep it moist, and smoke it as low as you can for as long as you can. Or you can just fake it. Applying a product like Morton’s Tender Quick will keep the surface pink because it contains both sodium nitrate and sodium nitrite. These are the same curing ingredients that keep ham and pastramis pink. It’s also what they used for that sous vide, oven-roasted, liquid smoke brisket at Chefsteps. Even brisket cooked in a plastic bag can have a rosy exterior, so think about that next time you judge a brisket by its smoke ring.

* Texas A&M has coursework dedicated to myoglobin explanation. Myoglobin within the muscle of a living animal is purple. When the muscle is exposed to the air it bonds with oxygen to become oxymyoglobin. As the oxygen exposure persists, the myoglobin (which is an iron compound) turns from ferrous FE(ii) to ferric FE(iii). It’s oxidizing like rust, and losing an electron. That’s when it turns from oxymyoglobin (red) to metmyoglobin (brown). Harold McGee, in his excellent book On Food and Cooking explains it this way. “[W]hen oxygen manages to rob the iron atom of an electron and then escape, the iron atom loses its ability to hold oxygen at all, has to settle for a water molecule, and the myoglobin becomes brown.” To the rescue comes nitric oxide (NO) which can replace the oxygen (O) in the myoglobin molecule and preserve the red color. The new compound is called nitrosomyoglobin**. The myoglobin bond is much stronger to NO than O, so it remains red after prolonged exposure, and even after boiling (I tried it). After gaining this understanding, it’s hard to think of proteins that I’m cooking as “dead meat” any longer.
**Carbon Monoxide can also bond to myoglobin in the same way that nitric oxide does*** with the same results to form a compound called carboxymyoglobin. It’s sometimes used as a food additive to keep meats pink or red. CO is produced by a wood fire, but according to Dr. Savell at Texas A&M, there’s little chance that CO would condense on the surface of meat in a smoker where O is also present.
***From Terrence Neumann, Ph.D of Texas Wesleyan University’s chemistry department: “Nitric oxide will bind about 100,000 times tighter to the iron than oxygen will. Carbon monoxide will bind about 2 times tighter to myoglobin than oxygen will.”