Sitting in his Rice University lab back in 2013, Jordan Miller was feeling stuck. The bioengineering assistant professor had spent months trying to do something no one had ever done before: create artificial lung tissue that functioned just like the real thing. And though he’d overcome some serious obstacles along the way, he’d reached one he couldn’t see his way past.
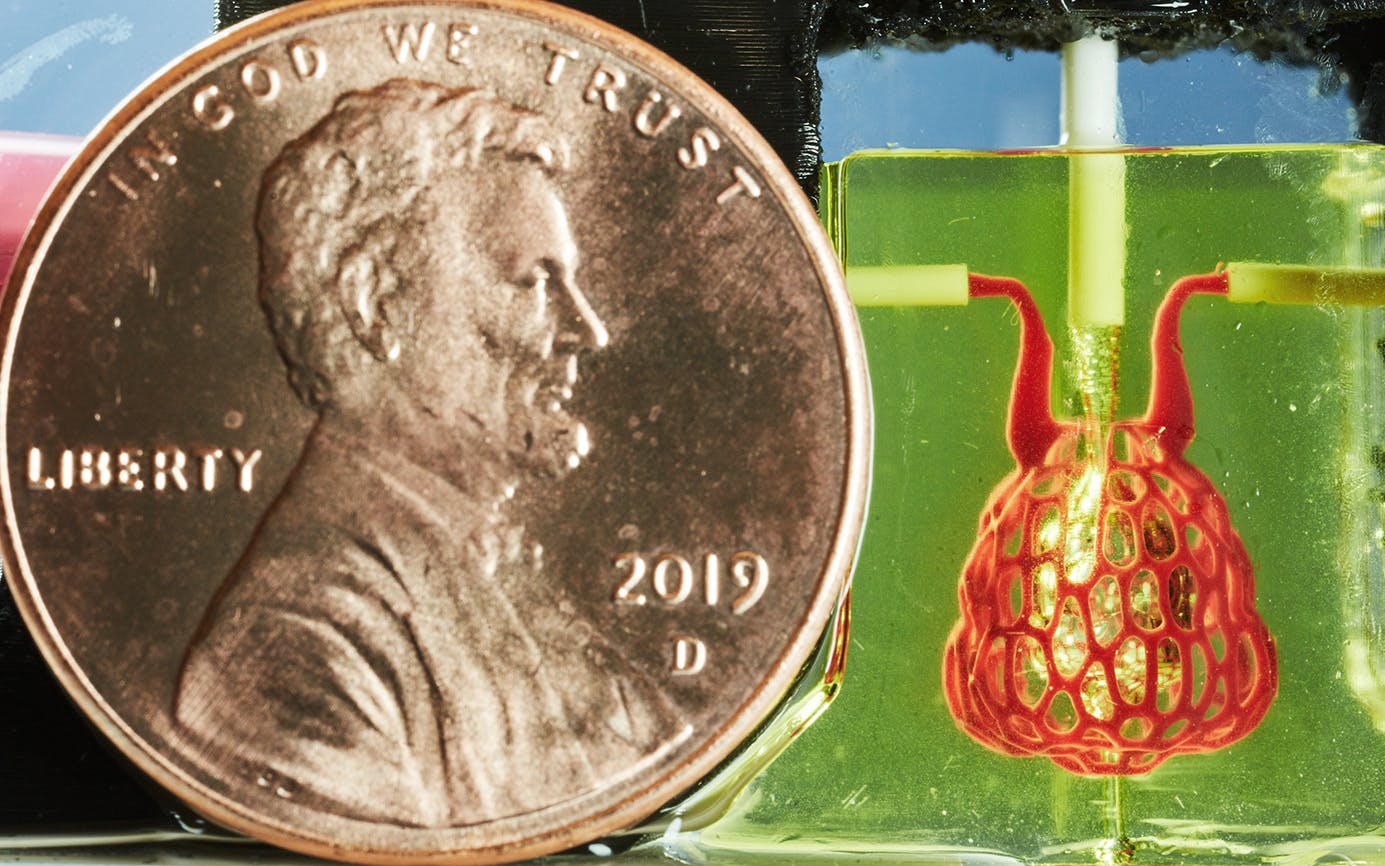
The process behind Miller’s experiment was, if not quite simple, at least easy enough to explain in general terms. His intent was to pour a water-based solution into a 3D printer of his own design and then flash a high-resolution image at the liquid from below. In theory, the light would, in a process known as photolithography, solidify the bottom of the liquid into a flat layer of gel, as if Medusa herself had shot her transforming gaze at it. Then the operation would repeat over and over, creating a series of stacked gelatinous layers that would form a biocompatible piece of tissue-like material.
There was a problem, though. To get the solution to solidify, Miller had to shine the printer’s blue light onto a yellow solution—no other color was as efficient at absorbing blue light. But Sudan 1, the standard yellow dye used in a photolithography 3D printer, doesn’t dissolve in water. What’s more, it’s toxic and carcinogenic—not exactly the kind of material people would want surgically implanted into their bodies. The only other dyes that Miller knew would work in the printer were toxic too. He was stymied.
Brainstorming with graduate student Bagrat Grigoryan, Miller asked a question that he probably didn’t need an advanced degree to pose: “What about food coloring?”
It was such a blindingly obvious suggestion that they were immediately skeptical of it. But they couldn’t think of any reason it wouldn’t work. After a quick visit to a nearby H-E-B, Grigoryan added some food coloring into the lab’s special mixture, turned on the printer, and the two men listened to the machine hum softly as it shone blue light on the yellow liquid and—zap! zap! zap!—created the first microlayer. Forty minutes later, when the printer signaled that it was finished, Grigoryan held up the piece of tissue-like material for his boss to inspect. “It was definitely a wow moment,” Miller says.


It was also the first in a series of victories. About a year later, using the same materials and a more complex version of the same process, a team of bioengineers led by Miller created an artificial version of an air sac, the part of a human lung that oxygenates blood and is essential to breathing, and therefore to life. The squishy, gelatinous, ten-millimeter-long air sac looked like a tiny, clear, bulbous purse, and when Miller and his team tested it, they saw that it could absorb oxygen.
It was an important step toward a major objective: creating artificial organs, which could save the lives of thousands of people a year. Achieving that feat remains far down the road, but this new process—enabled by simple grocery-store food coloring—has offered bioengineers a map for getting there.
The implications of Miller’s breakthrough could extend to virtually every organ in the human body. About 113,000 Americans are waiting for organ transplants from donors, and many of those transplants will require that recipients take powerful and dangerous drugs to stop their bodies’ immune systems from rejecting them—and sometimes they’re rejected even so. If the production of artificial tissue (and eventually full organs) can be perfected and scaled up, the dangers of long waits for transplants could all but come to an end.
It’s an exciting prospect that has attracted millions of dollars of government money, including from the U.S. military, and spawned private companies with names like Advanced BioMatrix, Cellink, and FluidForm. But organs on demand won’t be a reality anytime soon. And getting there will take humanity down some very strange, seemingly science-fictional paths.
Though more than 100,000 U.S. patients are awaiting transplants, the actual need for organs is even more dire than that number suggests; nearly 730,000 Americans die each year of organ diseases. Some researchers have suggested that a flush supply of organs would prevent more than 30 percent of all deaths in the United States. Barring that, we make do with what we have: generous organ donors and the best immunosuppressant drugs money can buy.
Attempts to build artificial organs from manufactured tissue to meet this demand are nothing new; the idea has been a research focus for the past few decades. In the nineties, Harvard University bioengineers hand-stitched a scaffold—the hollow structural framework—of a bladder from biocompatible materials and successfully grew cells derived from a patient’s own cells on it. They started with the bladder because it’s a relatively simple organ—basically just a muscular sac for holding urine with valves for releasing it—not nearly as complicated in its function as the kidney or liver.
By 1999, the Harvard researchers were implanting young patients with lab-grown bladders that continue to perform well. (Though, two decades on, engineered bladders still haven’t been approved by the FDA for widespread use.) The potential use of printers in this process was pioneered in 2000 by Thomas Boland, a researcher then at Clemson University who filled the ink tanks of a standard desktop printer with bio-inks—cellular material suspended in a solution—and created cells. Soon other engineers were using 3D printers to build layer upon layer of tissue.
At a much-ballyhooed TED Talk in 2011, Anthony Atala of the Wake Forest Institute for Regenerative Medicine, who was part of the Harvard team that created the artificial bladder, garnered press attention when he revealed onstage what many at first mistook for a functional human kidney that his lab had printed. It was, in fact, just a detailed model. It lacked the complex vasculature—the intricate arrangement of blood vessels vital to living tissue—necessary for an organ as complex as the kidney.
For years, bioengineers working with bio-inks failed to replicate the complexities of the multivascular networks found in our organs. By pursuing a photolithographic approach, Rice succeeded where everyone else had come up short. “That’s a nice advance because it addresses one of the main challenges we have today in the field, to bring vasculature and blood supply and oxygen and nutrients to the tissues being printed,” Atala told Texas Monthly. His lab has since experimented with the same technique.

Two years ago, when Miller and Grigoryan realized they were on to something, they founded their own company, Volumetric, looking to commercialize the technology they created, which now powers a 3D Printer called Lumen X. (Rice owns the intellectual property they developed while working at the university, and Volumetric has exclusive license for its use.)
Miller was inspired to pursue a career in tissue engineering as an undergraduate at Massachusetts Institute of Technology in 2001, after attending a professor’s talk about the potential for using skin scaffolds to aid in the healing of burn victims. “It was preventing scar tissue from forming and was saving people’s lives. It was a fascinating technology,” he says. Soon after, he asked to join the professor’s lab. He went on to earn a bioengineering PhD from Rice, followed by postdoctoral work at the University of Pennsylvania. In 2013, he returned to Rice, where he’s among a handful of professors working on regenerative medicine.
At age 39, he possesses a boyish quality; he talks about the body with enthusiasm, reverence, and awe, as if he’s discussing an architectural marvel. “You have a beautiful airway,” he says excitedly, “ensheathed by a very complicated blood vessel structure.”
One topic that particularly animates him is the notion that he may not need to draw exclusively on human biology to create artificial organs for humans. “Do we need to make something that’s identical to the organs in the body, or is something that’s functionally equivalent going to be as effective?” he asks. “It’s interesting.”
Actually, it’s kind of freaky. While the blueprints for organs will likely be inspired in large measure by human organs, they could also incorporate elements from other species. Human lungs are efficient, Miller points out, but they’re not as efficient as, say, bird lungs. And bird lungs aren’t as efficient as bat lungs.
Miller isn’t suggesting that he’d give someone oversized replicas of bat lungs—only that he might borrow design elements from them. And it gets weirder still. Ideas for organ innovations could come from outside the animal kingdom as well. Some of the designs used in Miller’s air sac, for example, were derived from the vein patterns present in leaves. “We recognize that a lot of the structure in humans resembles the structure in trees: branching, tapering, bifurcation,” he explains. “Because our bodies share design patterns that we see in plants, we can use plant-derived algorithms to design architecture for human tissue.”
In fact, Miller says that he’s been looking beyond flora and fauna for inspiration. Much as a prosthetic leg’s running blade functions more like a spring than a foot, some elements of his artificial air sac were derived from the structure of waterfalls. “You have the tiny tributaries draining into the bigger vessels,” he says, “and there are ways to mimic those phenomena in the lab and in the computational side to get things that will resemble some other vascular architecture and hierarchies that we see in the body.”
For now, though, the Rice lab is focused on optimizing the design of its air sac (Miller’s team’s tiny model was still ten times life-size) as well as other organ subunits, such as the lobule of the liver and the kidney’s glomerulus. These subunits must be perfected before anyone can build a fully functioning artificial organ. They also might first be deployed as implants that could work as short-term “patches” to keep patients’ organs working as they wait for compatible transplants. Atala’s team has already done this in trials at Wake Forest with bits of printed skin, cartilage, and muscle, as well as the vaginal and urethral organs.
It’s important to keep in mind that bioengineers have largely been focused on an artificial organ’s scaffolding—they still have a lot to learn about how to grow the human cells that would fill out the scaffolding to create a complete organ. Still, Miller believes we could see entire artificial organs within the decade. (Though decades more of clinical trials will likely take place before they are approved for widespread use.) The timing depends, of course, on funding and how well human bodies take to these artificial structures. There’s no guarantee that artificial organs won’t be rejected much as transplanted organs sometimes are, though there’s hope they will have a higher success rate since they could be created from a patient’s own cells.
The prospect of such technology transforming countless lives has motivated Miller since age sixteen, when, already deeply interested in biology, he was asked to check the organ-donor box on a driver’s license application. It was the first time he remembers reckoning with the knowledge that there are many sick people waiting for others to die just to have a shot at extending their own lives. “That was quite transformative for my young mind at the time,” he recalls. “It really said to me, ‘This is something worth working on.’ ”
This article originally appeared in the March 2020 issue of Texas Monthly with the headline “The New Kidney in Town.” Subscribe today.
- More About:
- Rice University
- Houston











