Elaine Dilley and her husband live in Brazoria County, south of Houston, on an eight-acre lot with pecan and oak trees and a well-stocked pond. Her mother lives there too, in a little green house at the end of the Dilleys’ gravel driveway. Last year, at 89, Dorothy O’Connell was still fiercely independent, mowing her own yard and driving her candy-apple-maroon Cadillac—which she washed herself once a week with a soap bucket and a garden hose. But O’Connell had severe arthritis, and doctors could do little to help her. “She stayed in pain from the time she got up until she went to bed,” Dilley says. “And it just kept getting worse.”
Dilley has spent her whole life on the Gulf Coast, always in her mother’s orbit. She’s strong in her own right: in her twenties, she was one of the first women to operate a crane at the Dow Chemical plant in Lake Jackson. She eventually moved on to other jobs and raised a son. In recent years, though, her mother’s pain had come to preoccupy her. O’Connell had been through surgery and injections of lidocaine for pain relief, and she and Dilley both thought she was out of options. Then, in late 2017, Dilley happened to read a column in her local newspaper, the Brazosport Facts, about stem cells—cells that have the potential to serve as a repair system for injured or diseased tissue.
The author, Gin Crawford, was something of a local celebrity. In a twice-weekly column, “Conversations With Gin,” she would offer a mix of witty musings and household tips, always closing the Wednesday column with a recipe—for tater tot casserole, say, or sauerkraut cake. She had flown to Arizona with her daughter so that both could have stem cell injections, intended to relieve her leg pain and help her daughter’s aching knee. The stem cells had worked, almost miraculously, it seemed to Crawford, so she wrote a column about them. And another column. And another.

This caught Dilley’s attention. “When you read about it every day,” she says, “it gives you hope.”
Crawford cautioned her readers to do their own research. She warned that stem cell injections weren’t cheap and weren’t covered by insurance. Yet she knew that some readers might seek treatment and wouldn’t be inclined to fly to Arizona, so she noted that stem cell injections were offered by a clinic in Missouri City, just southwest of Houston. The clinic, Texas Regional Health and Wellness, later bought ads in the Brazosport Facts that highlighted Crawford’s mention of the clinic in her column. (According to Crawford, she never gave permission to use her name in the ads.)
“Feeling better means more to me than money,” Crawford wrote, and Dilley agreed. If it would help her mother feel better, she would’ve been prepared to sell her house. So Dilley made an appointment for O’Connell at the clinic, where a chiropractor recommended stem cell injections, at a cost of about $17,000. Dilley took out a loan to pay the bill. Her mother had a few injections into her shoulders, then was told that she should also have shots into her back, which would be administered by an affiliate, a pain-management specialist she’d never met in an office she’d never seen, in west Houston.
On a Wednesday afternoon in September 2018, Dilley and her mother arrived at the Houston clinic and, while in the waiting room, chatted with the other patients. They learned that a few others were also from Brazoria County and had read about stem cells in “Conversations With Gin.” They’d come seeking relief from neck pain, back pain, and arthritis. One by one, they were called back to an area where they sat in recliners and were handed frozen vials, about the size of a paper clip, which they were instructed to warm in their fists. The contents were then transferred to a syringe and injected.
It was almost dinnertime when Dilley and her mother began the hour-plus drive back to Brazoria County. They stopped for a hamburger along the way, and once they were home, Dilley helped her mother to bed.
The next morning, around five, O’Connell called her daughter to say she felt sick. Dilley raced over and found O’Connell slumped in a chair, her head in her hands. She was so pale that her skin looked almost translucent. Dilley helped her back to bed, but her condition deteriorated. By the next day, Friday, even the slightest touch made her cry out in pain. Texas Regional Health and Wellness eventually prescribed Tylenol 3, a combination of Tylenol and codeine, but the pills did little to lessen her agony. By Saturday night, she was sweating heavily, vomiting, and babbling incoherently about her late husband. Finally, early Sunday morning, Dilley called an ambulance. “It didn’t look like my mother,” Dilley says. “It looked like a little frail old lady that would break if you would touch her.”
When the ambulance arrived, the paramedics were unable to fold up the gurney’s wheels and bring it inside the house. Dilley and the medics had to haul her mother outside in a bedsheet. “She screamed bloody murder the whole time we were moving her,” Dilley says. “I’ve never heard anybody scream like that.”
The closest hospital was in the town of Sweeny. Doctors there told Dilley that her mother was on the verge of a heart attack and that her kidneys were shutting down; they recommended she be airlifted to Houston. Dilley wasn’t allowed to ride in the helicopter, so she hurried to her car, stunned at the sudden turn of events. The highway was clogged from road construction, and the drive took much longer than it should have. When she finally arrived, she found her mother in the intensive care unit, hooked up to machines. She couldn’t even get near the bed because of all the medical personnel in the room.
The next morning Dilley received a text from the chiropractor’s assistant. At first, the message made no sense. It referenced two names she didn’t immediately recognize. Then Dilley realized the message wasn’t meant for her. It was meant for the assistant’s boss—and the names belonged to patients she’d met five days earlier in the Houston clinic, as they’d all waited for their stem cell injections.
A doctor was in the room with Dilley and her mother when she read the text. “I asked him if anybody else has gotten sick off the stem cells. He just looked at me,” she says. She wondered if there were others at the same hospital. “I could tell by the way he was looking at me, and the way he was shaking his head, that there was.”
Stem cells entered the public consciousness in the early 2000s, after scientists learned how to create stem cell lines for medical research using donated embryos left over from in vitro fertilization. At the time, researchers were enthusiastic about the promise of these cells. They still are. Some of the most serious forms of human disease arise when cells and tissues in the body wither and die—whether they be cartilage in joints, neurons in the brain, or overworked heart muscle. Stem cells were billed as would-be replacement parts, biological shape-shifters that might be transplanted and grow into whatever the body needed.
Yet even as scientists touted the medical potential of stem cells, antiabortion groups opposed the use of embryos, and in 2001 President George W. Bush banned federal funding for the creation of new cell lines—those not already in laboratories for research. (That moratorium lasted until 2009, though new restrictions were recently put in place by the Trump administration.) Paradoxically, the debate had the effect of publicizing the enormous promise of stem cell therapies, helping to create a receptive market for treatments that would eventually become available.
The cells advertised today in newspapers, on Facebook, and in the pages of in-flight magazines do not come from embryos but from adult bone marrow and fat tissue as well as a newborn’s umbilical cord blood, amniotic fluid, and other birth tissues. After decades of research, scientists are still optimistic that cellular therapy might one day treat a host of diseases. So far, though, stem cell treatments have been demonstrated effective and approved to help tackle only certain blood disorders, including some cancers. All other uses remain unproven. Yet their experimental status has not impeded a boom in sales. According to one study, stem cell treatments now constitute a $2 billion global industry.
Leigh Turner, an associate professor at the University of Minnesota’s Center for Bioethics, has been studying direct-to-consumer stem cell treatments for about a decade. He began with an interest in medical tourism; some Americans were spending tens of thousands of dollars each on international travel for stem cell treatments. Then, in 2012, he became aware of a new Houston company called Celltex Therapeutics, which had connections to a South Korean business he’d been tracking. Celltex could not have had a better political pedigree. Among its first customers was Rick Perry, then the governor of Texas, who received stem cell injections in conjunction with spine surgery. He later served on the board of the company until his appointment as U.S. Secretary of Energy. His wife, Anita Perry, currently serves on the board. Celltex has continued to align itself with the state’s elite, treating legendary former Texas A&M football coach Jackie Sherrill and former Dallas Cowboys superstar Tony Dorsett.
While the FDA passively allowed stem cell businesses to operate, the Texas Legislature actively sought to further promote them.
Turner urged the Food and Drug Administration to investigate whether Celltex, by administering an unapproved therapy, was violating regulations that protect patients. (Generally speaking, the FDA has stipulated that human tissue can be used in patients so long as it is “minimally manipulated”—a skin graft, for instance—and is intended for “homologous use,” meaning the extracted tissue serves the same function in the patient’s body as it would in the donor’s body.) In 2012, the FDA sent Celltex a warning, saying that the company’s practice of extracting a person’s cells and growing more of them in the laboratory put the treatment outside the bounds of “minimally manipulated.” Still, the company continued to thrive—by moving its clinical operations to Cancún.
In subsequent years, the FDA’s efforts to enforce the rules proved weak, Turner says. Providers figured out how to operate just inside the margins or simply ignored the regulations. Starting around 2014, the market exploded, Turner says. “I think part of it is [the FDA’s] not being that quick to understand how rapidly this market was moving, how far they were being outpaced by the proliferation of businesses. And then I think it was just basically being overwhelmed and being overrun.” (In a written statement, the FDA said, “We remain concerned that countless clinics across the country continue to market violative stem cell products to patients, claiming they don’t fall under the regulatory provisions for drugs or biologics. This is simply not true.”)
While the FDA passively allowed stem cell businesses to operate, the Texas Legislature actively sought to further promote them. So-called right-to-try laws, originally scripted in 2014 by the Goldwater Institute (a Libertarian-leaning think tank based in Phoenix) and since passed in more than forty states and federally, allow patients with terminal diseases access to experimental medicines that have passed basic safety tests in clinical trials, even if their effectiveness has not been proven. In 2017, members of the Texas Legislature decided to tailor the right-to-try concept to stem cells. State representative Tan Parker, a Republican from Flower Mound, says he became interested in stem cells because his wife suffers from lupus. He sponsored House Bill 810, which aimed to expand the right-to-try concept to “chronic conditions” and to treatments that had not gone through any clinical trials.
Although a coalition of scientists and patient-safety advocates called Texans for Cures nearly prevented the bill from reaching the House floor, they were outmaneuvered by its supporters. Just before a critical deadline, Representative Drew Springer, a Republican from Muenster, gave a tearful speech, saying that he hoped that stem cells would one day help his wife, who has a spinal cord injury. The bill ended up passing unanimously, giving Texas arguably the most lenient stem cell laws in the country. Almost immediately, says Kirstin Matthews, a fellow at Rice University’s James A. Baker III Institute for Public Policy, “we saw all these big, huge advertisements start popping up around the state and newspapers, TV ads, billboards promoting all kinds of stem cell interventions.”
“There’s no doubt in my mind that around the nation, stem cell manufacturers were watching House Bill 810 very, very closely and were so excited when it passed,” says David Bales, the founder of Texans for Cures.
A cursory Google search turns up video testimonials promoting stem cell treatments for everything from baldness to Lyme disease to autism. Many patients who receive the injections say they feel better afterward. But in the absence of conclusive studies, it’s hard to know whether the improvement comes from something in the cells themselves or from the considerable power of the placebo effect. Studies have found that the more expensive a treatment, the stronger the placebo effect—and patients are paying thousands of dollars for stem cell treatments.
“People who are desperate and have an incurable disease, they have a capacity to suspend disbelief,” says Sean Morrison, a stem cell researcher at the University of Texas Southwestern Medical Center, in Dallas, and the former president of the International Society for Stem Cell Research. “So when they go to these places to get these unproven therapies, they want to believe that they are going to benefit from it. It’s quite common for people to come back from those clinics to do these testimonials.” Because the providers aren’t being tracked as they would be in a clinical trial, there’s no data demonstrating actual results, but many people likely still succumb to their diseases, he says, “and you don’t hear about it on the company websites.”
By the time Dilley and her mother showed up at Texas Regional Health and Wellness, in 2018, the near-mystical allure of stem cells, fed by abundant advertising, had generated widespread demand. The biggest sellers are treatments for orthopedic conditions, due in large part to the prevalence of knee osteoarthritis among older Americans. More than 600,000 knee replacement surgeries are performed every year, but no one wants to undergo the expensive operation unless it’s absolutely necessary. Stem cells, clinics promise, can instead repair damaged cartilage with a quick injection.
While cells taken from the patient’s bone marrow or fat must be extracted in a doctor’s office, products made from donated umbilical cord blood or other birth products, which are sold in ready-to-use form by private companies, are easily administered via a quick injection. The procedure, which takes less than ten minutes, often costs a patient $5,000 or more. And since there are no requirements that the provider use special techniques and lab equipment, as there are for cells taken from bone marrow or fat, it’s easy to turn these particular stem cells into a lucrative line of business.
When she first brought her mother to Missouri City, Dilley didn’t realize that Texas Regional Health and Wellness was owned and operated by a chiropractor rather than a licensed medical doctor. Neither did the other patients Dilley met in the waiting room that day, among them Galen Dinning, a Dow Chemical retiree from Lake Jackson, and Deborah Williamson, also retired, a former teacher who was spending her life savings on the treatments. Dinning was plagued by severe neck pain. Williamson had pain in her back. They all were under the impression that the treatment would at the very least be safe, because it was being provided by a medical professional. Malpractice attorney Hartley Hampton, of Houston, says they had no way of knowing otherwise. He himself knew little about stem cells before he took on the cases. “I certainly didn’t know anything about umbilical cord blood stem cells,” he says. “I thought this was perhaps just a one-off adulterated batch, and then we got into it. We realized that there’s a systemic problem.”
The chiropractor who owns Texas Regional Health and Wellness, Sammy Tao, has been practicing since 2002. He started offering stem cell treatments in 2017 because he’d heard they were effective, he says, adding that his initiative was not related to the change in Texas law that year. After Gin Crawford mentioned his clinic in her column and he bought the ad in the Brazosport Facts, more patients began driving up from Brazoria County. Most of the injections are administered in his office by his nurse practitioner, he says, but for shots into the spinal column, he has partnered with the pain specialist that Dilley and her mother saw in Houston.
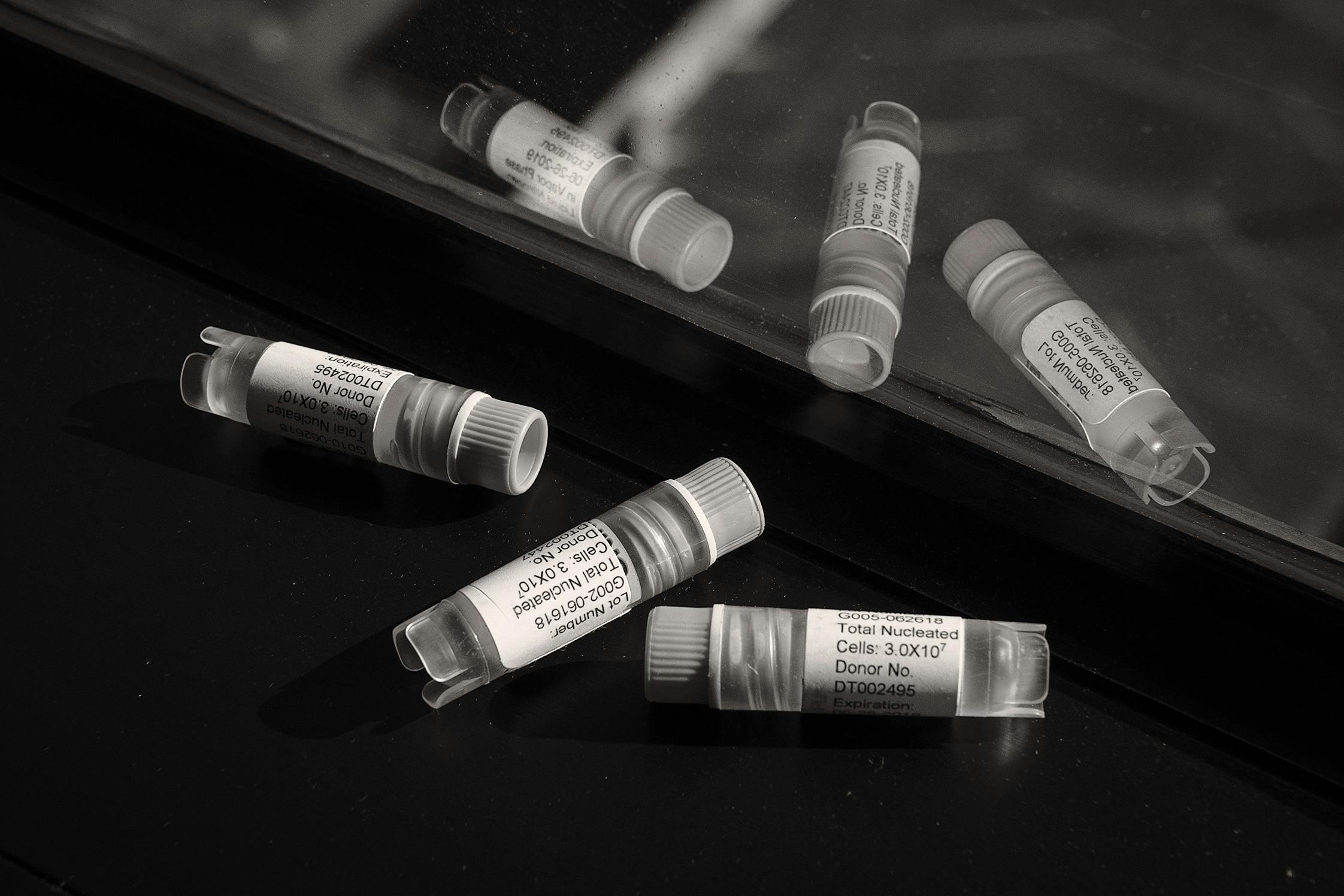
Tao purchased most of his stem cells from a company called Liveyon, which is based in Southern California and claims to be “the number one regenerative medicine company in the country.” Tao considered Liveyon’s product to be of higher quality and to yield better results than its competitors in the marketplace. And for almost a year, he says, he had no problems.
That changed the weekend of September 14, 2018, when he began getting frantic calls notifying him that three of his patients were in intensive care. “We heard about one, then two, then three,” he says. Dorothy O’Connell had arrived by helicopter in critical condition. Dinning had a fever of 106. Williamson was rushed in by ambulance from Lake Jackson. After a series of tests and scans, doctors discovered the source of the problem: all were suffering from life-threatening bacterial infections.
“I remember that was the most stressful time, because I literally was on the phone with Liveyon every day, a couple or three times a day,” Tao says. “We were praying. We never intended [medical complications] for anybody.” Tao tried to figure out what had happened. “At first, we were looking at, Was it possibly the procedure? Did we not have a clean environment? I keep questioning Liveyon. They keep denying there was anything wrong with the stem cells.”
In all, at least seven patients in Texas in the summer of 2018 suffered bacterial infections from stem cell injections. “I’m used to representing people who have been the victims of terrible medical accidents, and I’m used to dealing with people who are very, very seriously injured, but the thing that stood out to me about these folks is the degree of pain that they complained of and the vivid descriptions of how miserable they were,” says Hampton. “One of my clients was hospitalized for fifty-plus days, and he tells these stories of hallucinations that were so vivid and so serious that he developed post-traumatic stress disorder.”
O’Connell spent two weeks in the hospital and another six weeks in rehab, learning to walk again. She still suffers from damage to her eyesight and hearing, as well as vertigo. Dinning and Williamson also survived but are in more pain than before their stem cell injections. And the problems weren’t just in Texas. Patients in several other states also became ill from contaminated stem cell products. State and federal investigators sought to figure out where the contamination had come from, combing through records, sending leftover product for lab testing, and interviewing medical personnel. The trail led them to Liveyon.
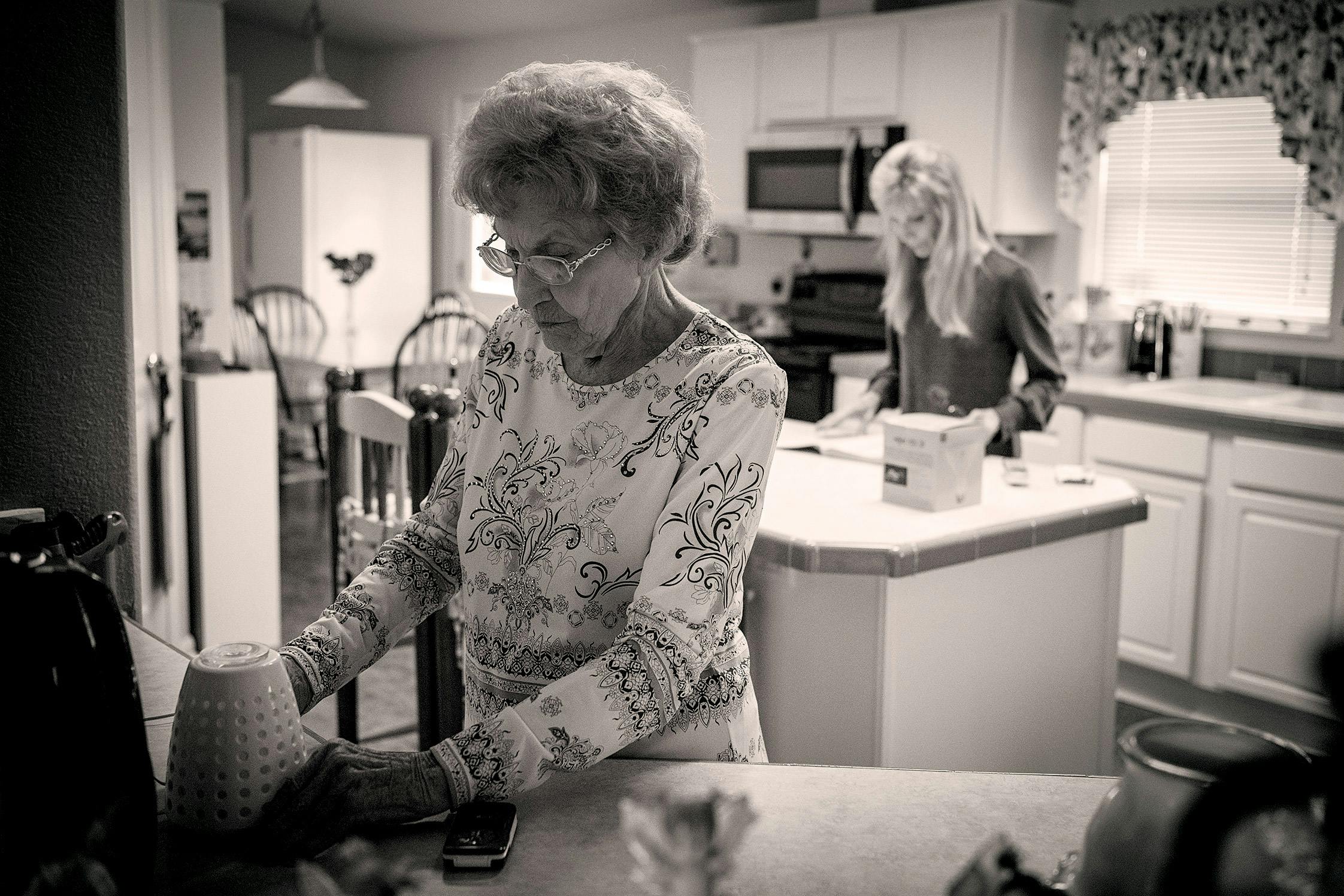
Success had come quickly to Liveyon. The company’s story begins with a 2013 Super Bowl party in Arizona, where a California businessman named John Kosolcharoen (pronounced “Co-soul-sharon”) smashed his knee during a poorly executed jump into a swimming pool. He returned home to Orange County on crutches. Lacking insurance at the time, he decided to try stem cells as a cheaper alternative to surgery. He visited a Beverly Hills clinic for stem cell treatment, and within weeks his pain subsided. A couple of years later, he flew his mother to California so she could receive stem cell injections for a burn on her hand. Not only did her hand heal, he said, her arthritis gradually vanished as well. Awed by stem cells’ apparent effectiveness, he decided to get into the business.
Kosolcharoen is a big guy, with thick black hair that’s always combed back perfectly in place. He was not new to the art of medical salesmanship. In 2013, he was reprimanded by the U.S. Securities and Exchange Commission for his peripheral involvement with a Dallas-based health insurance company that operated as a Ponzi scheme and bilked investors out of $10 million. The two people who ran the scheme pleaded guilty to wire fraud and were sent to federal prison. Kosolcharoen was not charged with any crime but was described in the SEC’s complaint as having “parroted the false claims” of the company to potential investors, and as a result, he was permanently barred from selling securities. (He says he was kept in the dark by the scheme’s ringleaders in Texas and thought the business was a legitimate investment opportunity for clients: “I had my whole family invested,” he says. “We all lost our money.”)
Then, in 2016, Kosolcharoen was arrested in a federal crackdown on health-care fraud. He’d been urging doctors to prescribe made-to-order pain creams to active and former members of the military at an average cost of $14,000 apiece. According to a complaint by the U.S. Attorney’s Office, Kosolcharoen was encouraging service members to seek prescriptions, receiving commissions from the compounding pharmacy that made the cream, and recruiting others to find him more patients. In 2015 alone, the pharmacy billed the government, through Tricare, an insurance program for the military, for almost $14 million in pain creams. Kosolcharoen attributes his arrest to aggressive prosecutors who leaned on unduly complicated military insurance rules and solicited the testimony of a former girlfriend. He says he pleaded guilty because otherwise he would have been charged with one count of fraud for every single prescription or “9,700 counts, which was a million years in prison.” His sentencing is currently set for May 2020.
Four days after his arrest, Kosolcharoen signed an agreement with a San Diego biotech startup called Genetech (not to be confused with biotech giant Genentech), founded by a young scientist named David Aguilar, to make a stem cell product from donated umbilical cord blood. Although Kosolcharoen says he intended to eventually build his own lab and manufacture the product himself, he partnered with Genetech so he could start selling the product more quickly. (Aguilar declined interview requests.)
Aguilar, in turn, contacted Jim Hardy, a longtime drug industry consultant who helps companies comply with FDA regulations. Hardy was based in Thailand, but he agreed to help remotely as Genetech’s director of compliance. Hardy holds a patent on a method to extract stem cells from a placenta, and he thought a startup like Genetech could help him commercialize his idea. He agreed to work without pay until the company was on its feet, figuring that it would take nine months to a year. But shortly after he signed on, in the fall of 2016, he was told that Genetech already had a customer that would sell and distribute the product to doctors.
“As soon as I saw ‘Liveyon,’ I’m like, ‘Oh, crap,’ ” Hardy says.
“I’m like, “ ‘First customer? We don’t even have a lab yet,’ ” Hardy says. That customer was Liveyon. When Hardy took a look at its website a few weeks later, he was taken aback. The site included a link to an advertisement for the product Genetech was making, with the slick production quality of a Hollywood movie. Even more concerning was a tab that listed a host of conditions that stem cells could supposedly treat, including multiple sclerosis, Lyme disease, and Parkinson’s. And, Hardy saw, Liveyon named Genetech as its exclusive supplier. Until that moment, Hardy had understood that the product Genetech would be making was for research purposes only—not for use in humans.
“It was just too many sensational claims,” Hardy said. That day, he sent an email to Genetech leadership listing the statements on the Liveyon website that were “inaccurate, misleading and non-compliant.” He said that Liveyon should probably be reported to the FDA. He tried to set up a video conference to discuss the problem, but it never happened. Hardy quit. Hardy says Aguilar told him he was quitting Genetech too. Hardy figured that the entire company would probably implode before it even got off the ground.
He gave little thought to his brief association with Genetech until a couple of years later, in October of 2018, when he was reading a popular stem cell blog and saw that patients had fallen ill after receiving stem cell treatments and that Liveyon had recalled its product.
“As soon as I saw ‘Liveyon,’ I’m like, ‘Oh, crap,’ ” Hardy says. “Then, of course, I knew it was Genetech, and I said, ‘Oh, crap.’ ” He was surprised not that something disastrous had happened but that Genetech had moved forward at all. “I thought they would have gotten caught sooner,” he tells me. But he has an explanation for why they were in such a hurry to get started. “People are making money all along the way.”
It’s difficult to tell exactly how much money is being made in stem cells, but the business is clearly lucrative and fast-growing. Liveyon’s tax returns reveal that the company brought in more than $6 million in sales in 2017, its first full year of operation. The next year, that figure more than doubled, to roughly $15 million. The company achieved that growth despite facing a recall of its flagship product—the one produced by Genetech—following the infections. It had to scramble to find another supplier. (Liveyon began distributing its own product, made in-house, in early 2019.)
Liveyon charges doctors $1,800 for a vial it says contains 30 million cells (although not 30 million stem cells), which they sell to patients for $5,000. I obtained an internal training call for its sales force, in which representatives are instructed to remind doctors that the vial can be used for more than one injection on the same patient—$5,000 for one knee, with a “discount” price of $2,500 for the second knee—increasing the doctor’s markup to $5,700 for a couple of quick procedures. Just ten patients a month with a simple markup of $3,500 per injection, the sales representatives are told, can add $420,000 to a medical practice’s annual bottom line. Meanwhile, a Genetech price list shows that Liveyon was buying the vials it sold to doctors for $1,800 at a price of $250.
Free seminars touting the benefits of stem cell treatments have proliferated alongside the clinics. I’ve sat in on several, and they seem to come with a mixture of science, testimonials, and sometimes a free sandwich and chips. The attendees are usually older, often steadying themselves on canes. One evening in April, I attended a seminar hosted by a Liveyon sales representative and a family doctor who does not accept health insurance and has embraced alternative therapies as part of his practice. It was held in a conference room at a La Quinta hotel in Sulphur Springs, a town of 16,000 about an hour’s drive from the skyscrapers of Dallas. There were roughly thirty seats, and all were filled. Three more attendees sat in wheelchairs.
Throughout the hour-and-a-half-long presentation, the doctor, Darrel Pierce, and the Liveyon sales rep, Tim Martin, promoted the healing powers of stem cells. A nurse practitioner from a clinic that Pierce was partnering with at the time told the audience about a stroke patient whose symptoms had improved after she gave him stem cells mixed with mannitol, a substance to help them cross into his brain. And Martin addressed Liveyon’s product recall. He assured the attendees that disease investigators had determined that the contamination in September 2018 was the fault of doctors, one of whom, he said, had E. coli all over his office. Another doctor, the salesman said, had improperly stored the vials.
In fact, the Centers for Disease Control and Prevention had issued a report months earlier with a different conclusion. It stated, “[I]nitial investigation suggests that contamination occurred before distribution,” meaning before the product was shipped to doctors’ offices. Genetech closed shortly thereafter. Liveyon had been its sole customer. (I spoke with Pierce a few months after the seminar, and he told me he had since ended his association with Liveyon, partly because he himself read the CDC report. “I did more research into what I was just told and didn’t find the answers that I liked,” he says. Martin did not respond to requests for interviews.)
During the seminar, the presenters walked through the science as they understood it. They explained that stem cell extractions taken from bone marrow—the chief competition for the umbilical stem cell market—contain far fewer actual stem cells than Liveyon’s product. A bone-marrow extraction would contain 2,500 stem cells, he told the audience. Liveyon’s product contained 300,000. “It doesn’t take a scientist to know which one may be more effective,” Martin said.
Or does it? I took an audio recording I made in Sulphur Springs to Sean Morrison, of UT Southwestern. So as to avoid the appearance of speaking out against a private firm, he agreed to help, with one stipulation: he didn’t want to know which company the seminar was representing. Like many other researchers, he believes in the potential of stem cells. He’s devoted his entire career to studying them. He worries that a lightly regulated retail market stands to tarnish the reputation of legitimate research. And he worries that the industry could siphon off patients who might otherwise participate in controlled clinical trials but instead opt to try the treatment on their own.
But mostly he worries about the patients themselves. I played him the tape of the claim about the 2,500 stem cells versus the 300,000. He took a deep breath. “In real stem cell therapies, we do pay close attention clinically to numbers of stem cells,” he says. “But those are details that get worked out over years and years of clinical trials, based on substantial numbers of patients.” In other words, it may be true that the number of cells matters, but it’s a complicated question still being investigated. Nonetheless, an information packet from one Liveyon-associated clinic includes a “dosing chart” with recommended numbers of cells for knee injections, erectile dysfunction, autism, neuropathy, and a host of other conditions.
I played Morrison the tape mentioning all the conditions I’d heard discussed. Almost all of these are the subject of intense research, he said, but the evidence is still inconclusive. He told me it’s not even clear that some of the offerings being touted as stem cell products contain any stem cells. They may contain other kinds of cells, but not cells that have been shown to repair tissue, or they may not contain live cells at all.
“What do you say to the argument that we know it works?” I asked.
“The people who make those arguments are the people trying to rip people off,” Morrison explains. Even when biology suggests a plausible mechanism, you don’t know until you do the studies. Hormone replacement therapy was given to millions of women, until studies showed that doing so entailed more risks than had been previously understood. High doses of vitamin E were once thought to prevent cancer, until a large study for prostate cancer found that the supplement actually increased the risk of malignancy. In fact, 90 percent of experimental drugs that go into clinical studies because they look promising end up not getting approved.
And even when things go right, they may have gone wrong at first. That was the case with the lone type of stem cell therapy that has been proven effective, which treats blood disorders. But in the course of the research on that therapy, all the initial patients died of complications from the treatment, Morrison says. The researcher “had to go back to the laboratory and spend fourteen years trying to figure out how to do it safely and effectively,” he says. “So even when there’s a kernel of truth underlying the science, it can be very dangerous to go forward clinically without doing the proper research to understand how to do it safely.”
In 2017, a scientist from the Netherlands reviewed all the human studies that have examined the use of stem cells for osteoarthritis of the knee, one of the most commonly advertised treatments. He found the existing studies to be of poor quality, lacking a true control. Since then he has identified two clinical trials that compared the treatment to a placebo—and in those studies, patients who received a placebo injection of saline solution reported the same level of improvement as those injected with cells from their bone marrow.
Dorothy O’Connell, now ninety, still lives by herself. She only recently started to drive again. “I’m real slow,” she says. “I can do a few things. I can cook and do things like that. But as for cleaning my house, that would be a chore.” She doesn’t remember anything about her acute infection, except for a vague recollection of the roar of helicopter blades.
The Brazoria County patients who received the contaminated stem cells live in the shadow of what happened to them. Elaine Dilley, her mother, and the other two Brazoria County patients feel they were deceived. Dilley and Dinning believed that the treatments they were buying had already been approved by the FDA, when in fact they occupied a regulatory gray area. “The FDA is between a rock and a hard place,” says Morrison. “They get criticized on both sides. Some people criticize them for slowing down things getting to market. Other people criticize them when something makes it to the market and hurts some people in a way that wasn’t anticipated.”
“Texas has become a wild, wild West,” says David Bales, of Texans for Cures.
The FDA has responded only to written questions for this story. Peter Marks, the head of the FDA’s Center for Biologics Evaluation and Research, recently told an audience of health journalists that the agency “is playing a game of Whac-A-Mole” and focusing on the most dangerous actors in the stem cell industry. Lately, he said, the FDA has stepped up its enforcement efforts. Earlier this year, it achieved a major victory when a judge ordered a Florida clinic to stop using fat-derived stem cells. For reasons that are still unclear, a series of patients had suffered permanent vision damage after the cells were injected into their eyes.
The agency is also seeking an injunction against a chain of stem cell clinics based in Rancho Mirage, California. “There’s a long lineup of people out there that are just waiting to defraud desperate patients by selling fake therapies that don’t have the potential to benefit people,” Morrison says. “It’s the FDA that stands between us and all of that fraud.” Or at least that’s what it’s supposed to do.
Other officials are also cracking down. The Federal Trade Commission has targeted some clinics for making false claims. The New York state attorney general recently sued the owner of a Manhattan stem cell clinic, claiming that it was misleading and defrauding the public. And in what may prove one of the most meaningful actions, Google recently announced that it is banning all ads for unproven stem cell therapies.
State representative Tan Parker, who sponsored the law that expanded access to unproven therapies in Texas, says he wants stem cells to be administered safely while also giving ill patients the freedom to try something experimental. “I only want high-quality, reputable physicians that are doing good work providing these types of services to the people of Texas,” he says. He told me his bill was intended to allow doctors to grow a patient’s cells in the lab before injecting them—the procedure that had originally gotten Celltex into trouble and had prompted it to move its clinical operations from Houston to Cancún.
While Parker refers to “doctors” and “physicians,” the Texas Medical Board believes that they aren’t the only ones administering stem cell treatments and that some chiropractors are not following the rules. “Texas has become a wild, wild West,” says David Bales, of Texans for Cures. “We never, ever saw chiropractic clinics advertise to provide stem cell treatments until House Bill 810 passed.”
Last March, the medical board’s executive director sent a letter to the Texas Board of Chiropractic Examiners, emphasizing that the law requires stem cells to be administered by a physician. Patrick Fortner, the executive director of the Texas Board of Chiropractic Examiners, told me that while investigations are ongoing, so far the board has not found instances of chiropractors either administering stem cells or ordering others to administer them.
In August, Dilley accompanied her mother to a cardiologist appointment. While they were waiting to see the doctor, she overheard a nearby conversation. “There was a man talking to this woman that was sitting next to him, and he started talking about having stem cells done in his knee,” she says. Everyone else in the waiting room, she recalls, started to discuss all the wonderful things they had heard about stem cells. “I just could not keep my mouth shut,” she says. “I had to say something, and I started telling him, ‘Look, have you checked into this? Did you know they weren’t FDA-approved?’
“They were all interested in it, because they had not heard really any bad things about stem cells. They all just hear all this fantastic stuff.”
Everyone in the room listened to her story. Her mother sat beside her and nodded along.
Laura Beil is a science journalist based in Dallas. Her new podcast Bad Batch, from Wondery, is an extended investigation of the Texas infection outbreak and the pitfalls of the stem cell industry.







