Fourteen years ago in Birwadi, a town of less than 10,000 in west-central India, Ambalika Tanak’s grandmother suffered a heart attack. But her doctors didn’t know it. The 72-year-old’s symptoms were ambiguous, as is the case with many women experiencing a cardiac event, and the local hospital didn’t have the equipment to properly diagnose her. They mistakenly thought she was having an asthma attack, so she didn’t get the treatment she needed.
Small communities like Birwadi have fewer resources for assessing and managing complex medical conditions than do the country’s big cities. When Tanak’s grandmother’s condition worsened, the family decided to transfer her to Mumbai, a nearly five-hour drive away. By then she needed a ventilator to breathe. “If the countryside hospital had done an EKG, they would have known of her heart attack and started treatment right away,” Tanak said.
As she held her grandmother’s hand in the intensive care unit of the Mumbai hospital, Tanak took note of the medical equipment the older woman was hooked up to. “The nurses kept coming in to test her blood pressure and other vital signs,” Tanak said. “I realized how much the doctors rely on these devices.”
Then age sixteen, Tanak had just graduated from high school. She planned to study engineering, but she didn’t know exactly what kind of engineer she wanted to become. It was during her grandmother’s hospitalization that she realized her chosen field of study might be a way to save lives, by building better medical tools. “That was when I decided that biomedical engineering would be the best fit. As an engineer you’re able to contribute toward those real-life problems,” she said.
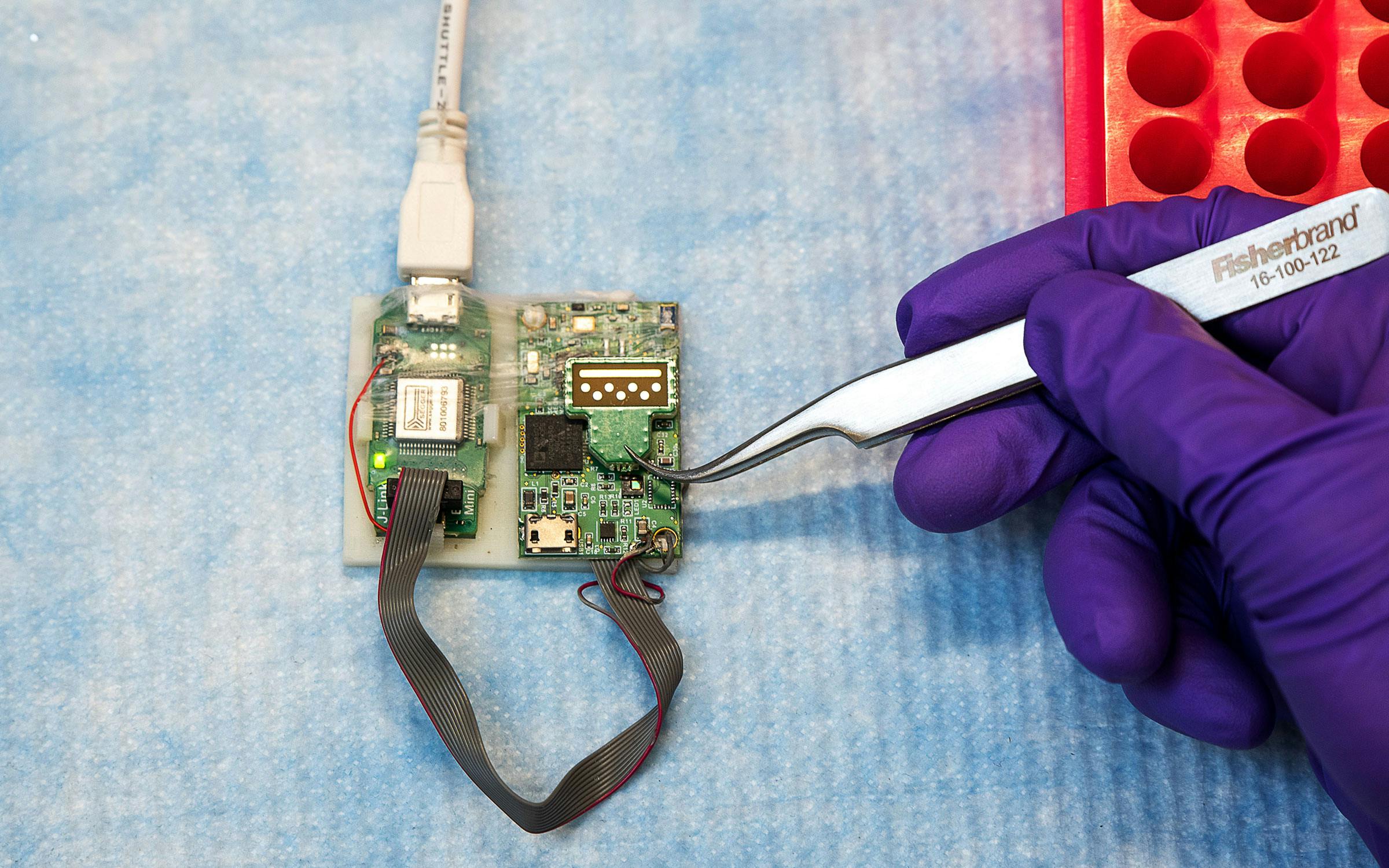
Today Tanak is doing exactly that, as a graduate student in biomedical engineering at the University of Texas at Dallas. She recently developed a biosensor that requires only a single drop of blood to give doctors a clinical snapshot of a patient with sepsis, a leading cause of death in U.S. hospitals and a condition that often goes undiagnosed until it’s too late. Though much work remains to be done before it can be put to use in hospitals and clinics, Tanak’s device might help doctors recognize sepsis much earlier, before the most severe symptoms begin to occur. That could save countless lives.
“There’s been a lot of research on sepsis, but the major thing missing was an active point-of-care testing device that can give feedback at the patient’s bedside,” Tanak said. That’s the gap she hopes the Direct Electrochemical Technique Targeting Sepsis biosensor (DETecT) will fill.
During sepsis, the immune system goes haywire in response to infection by a bacterium, virus, or other microorganism. Some of the body’s defenses shift into overdrive, others cease functioning, and chemicals get released into the bloodstream that cause inflammation throughout the body. If left untreated, sepsis can lead to severe tissue damage, the shutting down of one organ after another, and eventually death. “It’s not the pathogen causing you to die,” said Dr. Subramaniam Krishnan, one of the coauthors of Tanak’s proof-of-concept study of the biosensor. “It’s the dysregulation of the immune response.”
Detecting sepsis as swiftly as possible—even a matter of hours faster—can make a significant difference in whether a patient survives. “When you first recognize infection and have the early warning signs that the body is starting to respond to infection, that’s the time to intervene, not when the damage has already happened,” said Dr. Luis Ostrosky, the chief of the division of infectious diseases at UT Health Science Center’s McGovern Medical School and medical director of epidemiology at Memorial Hermann–Texas Medical Center, both of which are in Houston. “Timing is everything. The problem is that people don’t recognize this. There’s a point of no return when you cross a threshold where the damage caused to our bodies is irreversible, and people die.”
Sepsis plays a role in one in three hospital deaths in the United States, killing roughly 270,000 of the 1.7 million Americans who develop it every year, according to the Centers for Disease Control and Prevention. That’s only a fraction of sepsis deaths across the world. A study in the Lancet in January 2020 estimated that nearly 50 million patients developed sepsis in 2017 and that 11 million of them died, accounting for one in five of all global deaths that year.
Why the high death toll? Infections are common and the condition is complex, according to Krishnan, who’s also the chief science strategist for Austere Environments Consortium for Enhanced Sepsis Outcomes (ACESO), a group based in Bethesda, Maryland, whose goal is to improve survival rates among sepsis patients in remote areas that lack medical resources.
Treating sepsis is challenging because so much is happening in the body at once, explained Dr. Charles Lerner, a retired infectious disease physician who consults for the Texas Medical Association’s Committee on Infectious Diseases. “All kinds of things go on in sepsis,” he said. “Sepsis is the combination of changes in blood pressure and pulse, the immune system, the coagulation system, the ability to get oxygen through the lungs into the tissues, and, occasionally, diminished kidney blood flow.” Those are the primary physiological parameters doctors currently use to identify sepsis, along with rudimentary lab tests (of unproven efficacy) seeking certain proteins in the blood.
But sepsis, especially in its early stages, can sneak up on doctors who don’t suspect it, said Dr. Chandra Mohan, a professor of biomedical engineering at the University of Houston and an adjunct professor of medicine at UT Health Science Center in Houston. “It’s been described as a silent killer, meaning that we don’t even think of sepsis because the patient presents with just confusion or maybe a heart rate increase or breathlessness,” he said.
Tanak’s biosensor detects levels of five inflammatory markers that research has suggested are measurable indicators of the immune system’s response to sepsis. In Tanak’s study, published in January, the sensor identified those biomarker levels in twenty archived blood samples with at least 85 percent accuracy in just five minutes. Further testing will seek to demonstrate that it’s capable of the same diagnostic results with drops of fresh blood.
The UTD team hopes to expand the biosensor’s capabilities to measure seven or more markers. There are devices already in use that measure the type and severity of the underlying infection in a known sepsis patient, as well as look at cellular changes. But none of the available tools help doctors tailor their treatments. Though it has not yet been demonstrated in clinical studies, Krishnan said, DETecT’s measurement of the five biomarkers alone could help doctors determine if an underlying infection is bacterial or viral. Doctors would then know whether antibiotics should be used or avoided.
“Assuming it’s validated, this is a good step in the right direction,” said Dr. Faisal N. Masud, the medical director of critical care at Houston Methodist Hospital. But “if it doesn’t change outcomes, then we’ve actually added cost to the care of the patient.”

Tanak developed the sepsis biosensor by customizing technology developed by her adviser, Shalini Prasad, the head of UTD’s department of bioengineering, and Sriram Muthukumar, a UTD adjunct engineering professor. Prasad and Muthukumar cofounded the company EnLiSense specifically to develop biosensors and then partnered with ACESO to determine the greatest need in sepsis care.
Muthukumar realized the potential for this kind of product after speaking with military doctors. A U.S. Army officer described the challenge of being at an Army camp in Afghanistan and needing to know whether patients have sepsis—and can still be saved—before deciding to airlift them to Germany, at a cost of tens of thousands of dollars. “That defined the value of detecting sepsis in the field,” Muthukumar said.
Tanak initially created a sensor to measure parathyroid hormone levels, which could assist surgeons in determining more precisely what tissues should be removed during surgeries. She applied that experience to her sepsis sensor after spending months learning which inflammatory markers were most helpful in identifying sepsis. Doctors can already test these markers with standard lab work, but that usually takes at least a few hours (compared with five minutes for the DETecT sensor). Plus, such lab tests may not be an option for smaller hospitals. “Undoubtedly, point-of-care molecular biomarkers are the future, not only for sepsis but for identifying the infections that trigger it,” Ostrosky said.
Tanak’s work earned her the first-tier Baxter Young Investigator Award, an honor granted by the health-care manufacturing company Baxter to just six graduate students and postdoctoral fellows each year for innovative, potentially lifesaving research. “As a PhD student, you try to delve into the challenges that have not been solved,” Tanak said.
Her grandmother survived another week before a second heart attack ultimately caused her death, but Tanak still wonders if she might have lived longer had she gotten the care she needed faster. At the time, Tanak could only offer her grandmother comfort by holding her hand. Now she’s putting her hands to work in her lab, hoping to prevent others from losing their loved ones too soon.
Tara Haelle is an independent science and health journalist based in Dallas. She’s the author of The Informed Parent and Vaccination Investigation: The History and Science of Vaccines.
This article appeared in the July 2021 issue of Texas Monthly with the headline “Tracking a Silent Killer.” Subscribe today.
- More About:
- Health
- Richardson