Until he was three and a half years old, Joseph Hann didn’t seem so different from any other kid his age. He loved swimming, riding his balance bike, trick-or-treating at Halloween. Then he began bumping into things around his family’s home in Tucson, Arizona. His parents, Matt and Gina Hann, figured he was just a little clumsy or was running around faster than he should. Soon they discovered he could no longer identify letters of the alphabet on an eye chart he had in his room. Something was wrong with his vision.
Joseph got glasses, but no prescription seemed quite right. A trip to a specialist in Phoenix revealed a retinal abnormality, which suggested that Joseph was suffering from a degenerative eye disease. There was a good chance, the Hanns were told, that the boy would go blind.
Matt and Gina resolved to fill however many days of sight their son had remaining with as many vivid memories as they could. They booked a trip to Disneyland for Joseph and his three siblings. But a week before they were set to travel to California, Joseph was sitting in his dad’s lap playing with an iPad when he collapsed into a grand mal seizure, the muscles of his body violently contracting as he temporarily lost consciousness. It lasted more than thirty seconds. “He hit the floor,” Matt remembers, “and then he kind of woke up from it with that look of ‘What just happened?’ ”
They rushed Joseph to a nearby hospital, where an emergency-room neurologist thought he recognized the root of the boy’s problem. Just a couple weeks earlier, the doctor had read a paper about a rare genetic disorder of the nervous system. Hearing that Joseph wasn’t as agile as the other Hann children, was losing his sight, and now had suffered a seizure, the doctor suspected Batten disease. There are fourteen types of Batten, and all are fatal, nearly all in childhood. A test to look for mutations in Joseph’s genes would be necessary to determine whether the neurologist’s suspicion was correct, but the results wouldn’t be available for six weeks.
The Hanns didn’t just wait around for the diagnosis. They went ahead with the Disneyland trip. (“I was a mess,” Gina says, “but the kids thought it was the happiest place on earth.”) By the time they returned, they had decided to leave Tucson and move wherever Joseph could find the best care.
Dallas was an early front-runner. Gina and Matt are longtime employees of Texas Instruments and could transfer to work at its corporate headquarters there. Soon a chain of email referrals put them in touch with Dr. Berge Minassian, a pediatric neurologist who had joined the University of Texas Southwestern Medical Center only a few weeks earlier, in January 2017. A phone call with Minassian, who had worked on similar genetic disorders, sealed the Hanns’ decision. They’d pack up their four children and move to Texas in hopes that it would give their son a better shot at life.
By the time the genetic test results arrived, a few weeks later, the Hanns were closing on the purchase of a house in the prosperous suburb of Southlake. Joseph indeed had Batten disease—one of the rarest forms, known as CLN7. As the disease progressed, he would lose not only his sight but also his ability to walk and speak. He’d be lucky to live into his teenage years.
Minassian had come to Dallas for the specific purpose of trying to cure patients very much like Joseph. For twenty years, the native of Lebanon had happily worked at Toronto’s Hospital for Sick Children. But when UT Southwestern asked him to become its new head of pediatric neurology, he made a proposal. He wanted to build a gene therapy center, on the cutting edge of medical technology, where researchers and physicians could collaborate on treatments for a whole spectrum of genetic disorders. He was tired of attending funeral after funeral, frustrated about not being able to do more for patients. UT Southwestern had been expanding its research and treatment facilities in recent years, thanks in part to generous gifts from Dallas philanthropists, and jumped at the opportunity. “They saw that this is part of the future of medicine, which other places haven’t,” Minassian says.
UT Southwestern’s gene therapy center is expected to be fully operational by the end of this year. And the Hanns no longer have to simply accept that their son’s early death is a foregone conclusion. After Minassian saw Joseph’s test results, he of course told them the prognosis was bad. But now, with his center being built, he was able to add, “There’s hope, maybe.”
Steven Gray, a biomedical researcher at the University of North Carolina, was on Minassian’s mind when he spoke to the Hanns. Minassian was trying to recruit Gray, who had done some work on CLN7, to join him in Dallas.
Today, more than two years later, the men sit side by side at a long table in a windowless conference room on the UT Southwestern campus, at the end of a hallway lined on both sides by lab space that’s being transformed into the gene therapy center of their dreams. It’s a collaborative effort between Gray’s team of researchers and Minassian’s team of physicians. While there are a handful of other academic gene therapy centers in the country, most tackle just a few diseases. “Here we are bringing in pediatric neurologists to work with [Gray] on twenty or more different diseases,” Minassian says.
Guangping Gao, the president of the American Society of Gene and Cell Therapy, says an academic research institution operating at that scale would be “incredible.” He describes Gray as “very motivated. His goal is to clear rare diseases off the table.”
Indeed, UT Southwestern’s program could soon transform many lives. For much of modern history, doctors have classified diseases primarily based on their observable symptoms. A patient like Joseph, going blind and brought to an ER after a violent seizure, might well have simply been diagnosed as epileptic. That’s not incorrect; “epilepsy” is a general term for any brain disorder that causes seizures. But since the mapping of the human genome—the complete genetic blueprint for building a human—was completed, in 2003, researchers have been able to pinpoint the exact causes of a host of diseases, including the broad array lumped together as epilepsy. Joseph’s form of Batten disease, for instance, is known to be caused by a mutation in the CLN7 gene that results in a failure to produce a necessary protein, which in turn leads to developmental problems.
Most childhood genetic disorders can similarly be traced to a problem with a single gene, and that’s why curing such conditions seems so tantalizingly possible—even if scientists still can’t entirely explain the processes by which a specific defect alters the brain’s development. “If we could simply fix the cause, irrespective of our relative lack of understanding of how the brain works, we could help the patient,” Minassian says. “I really wanted to dedicate the rest of my career to working on ways to solve this problem. In so many other diseases, the problem is that we don’t understand the cause. Here we know the cause.”
The potential solution: in the simplest terms possible, doctors piggyback a healthy copy of a mutated gene onto a benign virus and inject that into a patient. The patient’s body, in theory, can then use the healthy gene to restore or correct whatever functions were affected by the defect. The brain degeneration resulting from conditions like Batten disease might thereby be arrested.
Recent years have brought encouraging evidence that this approach is far more than a pipe dream. Following a successful clinical trial, the FDA recently approved a gene therapy treatment for a devastating condition called spinal muscular atrophy, which typically kills infants. “Since humans came down from the trees, or wherever they came from, most patients with this diagnosis have not lived beyond the age of one or two,” Minassian says. “Now they’re up and about. It’s really weird and wonderful.” News spread this spring that eight infants born with the severe immune disorder commonly known as “bubble boy disease” had been successfully treated with gene therapy. And Gray himself organized a 2015 clinical trial, along with the National Institutes of Health, in which a girl with giant axonal neuropathy recovered some mobility that she’d lost.
It’s not only rare pediatric diseases that could someday be helped by a onetime treatment. Much more complex (and common) problems like Alzheimer’s, schizophrenia, and Parkinson’s, which involve multiple genetic mutations, might also be overcome. Researchers will learn much about the potential to do so by first confronting relatively simple childhood diseases. They are “our training ground to learn how we’re going to be able to get in the brain and manipulate genes to fix the huge diseases,” Minassian says.
Yet producing, in volume, viruses of the type and purity necessary to carry the healthy genes and implant them into patients’ cells requires a highly specialized facility. There are relatively few academic institutions and commercial labs capable of it, which often results in waits of as long as two years for a potential trial to obtain the required patient doses, at costs totaling millions of dollars per trial.
Minassian was able to recruit Gray to join him in Dallas by promising that the medical center would build its own virus production facility—which would give them more control over both project time lines and costs. When virus production gets underway later this year, Dallas will become a center for research into pediatric genetic disorders. Already, Minassian and Gray have more than six months’ worth of studies lined up. “I get contacted every other week by a new family about some disease,” Gray says. “We have people preparing to fly from Australia to come live here,” Minassian adds. “It’s happening.”
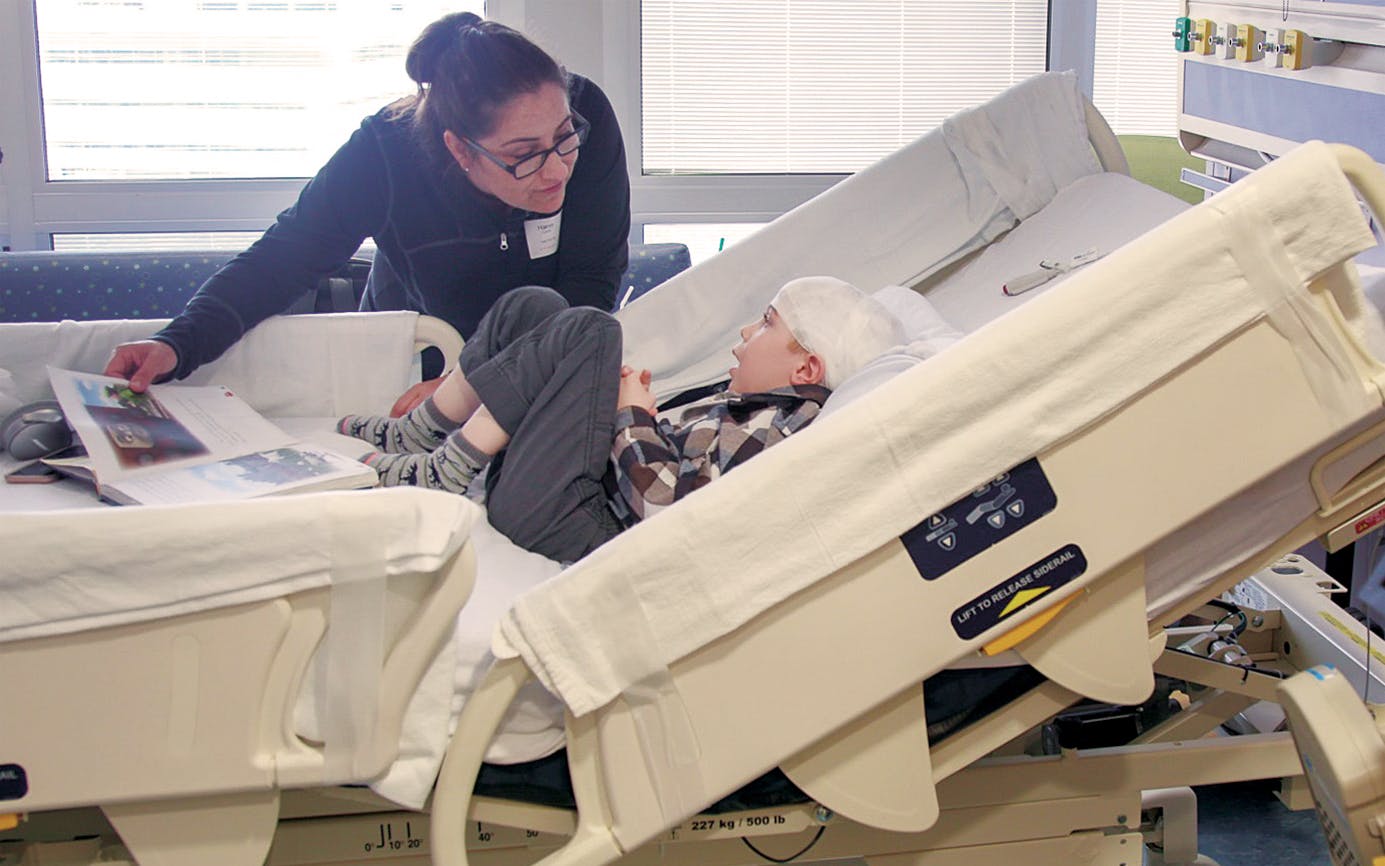
The Hann family couldn’t afford to wait for UT Southwestern’s virus production facility. The clock was ticking from the moment they got Joseph’s Batten diagnosis. He lost his vision within about a year, and shortly after that his motor skills degraded to the point that he could no longer walk on his own. Around the start of this year, he lost his ability to speak much beyond cooing or squealing.
The sliver of hope that Minassian offered the Hanns came at a high price. Because of the rarity of Joseph’s disease—there are no hard numbers available, but Minassian estimates 1 in 500,000 is born with it—funding for a gene therapy study was much harder to come by than it would be for more common diseases. If the Hanns wanted a clinical trial to test a potential cure for their son, they’d have to raise money—at least $1 million, they were told, possibly more. Insurance companies don’t pay for such scientific research.
After establishing a fund called Batten Hope, Gina and Matt began reaching out to corporations and foundations as well as their social circles. Within fourteen months they’d met their goal, and to date they’ve raised more than $1.5 million. (Minassian marvels that one of the unexpected benefits of having set up shop in Dallas is that it’s an excellent place to raise money.) The Hanns eventually connected with Vigene Biosciences, a company that could build the viral doses sooner than Gray could. If everything goes according to plan, UT Southwestern’s clinical trial on CLN7, using the Vigene doses, will begin later this year.
There’s no guarantee, however, that Joseph will be among the six to ten patients who will each have a quadrillion viral particles (a couple teaspoons’ worth) injected into their spinal fluid in an attempt to stop or slow their brain’s degeneration. There’s a possibility that guidelines for the trial, as set down by the FDA and UT Southwestern, may determine that he is no longer healthy enough to participate.
For Minassian and Gray, it’s hard to see a patient like Joseph—one they’ve come to know well—potentially denied treatment. Gray has become personal friends with the Hanns. But there are good reasons for running a study like this one strictly by the book. “If we move in an irresponsible way and, God forbid, something disastrous happens in the context of a clinical trial, it doesn’t just affect that patient. It doesn’t just affect our one trial,” Gray says. “It affects the whole field of gene therapy.”
The Hanns are painfully aware that Joseph might get left out. They choose to see their now seven-year-old’s journey as one of hope, not despair. Many children with rare diseases go misdiagnosed for years—and yet, after Joseph’s first grand mal seizure, the Hanns just happened to end up in an ER with a doctor who just happened to have read a paper about Batten disease. They just happened to find Minassian at the moment he was setting up a gene therapy center, and Minassian just happened to recruit Gray, who just happened to have recently done research on the very disease that Joseph suffers from.
The Hanns pray for the good fortune to continue, Gina says. As we chat, Joseph sits a short distance away, parked close to the TV. He can’t see the cartoons, but he still loves to listen along. Periodically he honks the horn attached to his walker to playfully chase off his younger sister. He’s a happy boy. “I have all the faith in the world this is going to come out in our favor,” Gina says. She also knows that the CLN7 trial is happening because of Joseph, and even without him in it, it could save other children’s lives. “That’s a pretty awesome legacy.”
This article originally appeared in the August 2019 issue of Texas Monthly with the headline “Two Teaspoons of Hope.” Subscribe today.